Information for researchers
Whether you are setting up your own study or want to participate in an existing study, we are here to help support you with every aspect of the research process.
Before contacting us, please ensure you read through all of the information below. If, having read the information, you have any specific questions or requests or you have heard about a study you are keen to be involved with, please email us and we will aim to get back to you within seven days.
IRAS is a single system for applying for the permissions and approvals for health and social care / community care research in the UK and captures the information needed for the relevant approvals from the following review bodies:
- Administration of Radioactive Substances Advisory Committee (ARSAC)
- Confidentiality Advisory Group (CAG)
- Gene Therapy Advisory Committee (GTAC)
- Health Research Authority (HRA) and Health and Care Research Wales (HCRW) for projects seeking HRA & HCRW Approval
- Medicines and Healthcare products Regulatory Agency (MHRA)
- NHS / HSC R&D offices
- NHS / HSC Research Ethics Committees
- HM Prison and Probation Service (HMPPS)
- Social Care Research Ethics Committee
If you are a first time user of the Integrated Research Application System, you will need to create a "New User" Account.
The Health Research Authority is responsible for Research Ethics Committees (RECs) and their website provides a valuable and useful resource when setting up your study.
Every research project requires a Sponsor and their role is to take overall responsibility for the management and monitoring of the study. Normally, the sponsor will be one of the organisations taking the lead for particular aspects of the arrangements for the study, such as the Chief Investigator’s employing organisation, the lead organisation providing health or social care, or the main funder (eg a University, Pharmaceutical Company or an NHS Trust).
The Isle of Wight NHS Trust may be willing to act as the sponsor for research that does not have an external sponsor (sometimes called “own account” research). If you would like the trust to sponsor your study, please contact the Research Department.
Written permission must be obtained from the Research Department before any research starts.
Every study is considered by the Trust’s Research & Development Department.
To complete and submit your submission to the R&D Office for approval, please refer to IRAS Checklists A1, A2, A5 and A6 for a list of documents that we will require to process your application, depending on whether your study is a CTIMP (Clinical Trial of a Medicinal Product) or not.
All documents would need to be dated and/or have version numbers and these can be sent electronically by email,
or by post to: Research Dept, Lower Maternity Corridor, North Block, St Marys Hospital, Newport, Isle of Wight, PO30 5TG
The trust is keen to develop and expand its commercial involvement across the specialties. The contribution of departments and individuals to commercial studies is recognised by a distribution of the income generated in line with NIHR’s good practice guidance, as detailed here in the Income Distribution from NIHR CRN Industry Portfolio Studies.
A key requirement for anyone involved in the conduct of clinical research is Good Clinical Practice (GCP) training. All research active professionals participating in research are required to undertake GCP training before commencement of recruitment for CTIMPs (clinical trials of investigational medicinal products) and within 6 months of R&D approval for all other studies (non-CTIMPs). This training is to be refreshed every two years for those who remain research active. Please complete GCP training here.
If you are interested in being informed about future studies, please email the Research Department, informing us of your job title and your areas of interest. We will log these and contact you in the future should we be made aware of any suitable research you may be able to become involved with.
The Wessex REACH initiative enables healthcare professionals to take research ideas from concept to reality. Developing treatment options and improving patient outcomes relies on new research taking place across all areas, and the Wessex REACH team are passionate about making this possible. For further information and training opportunities, please visit www.
The Associate Principal Investigator (PI) Scheme is a six month in-work training opportunity, providing practical experience for healthcare professionals starting their research career. For more information about this and how to apply, please visit Associate Principal Investigator (PI) Scheme | NIHR
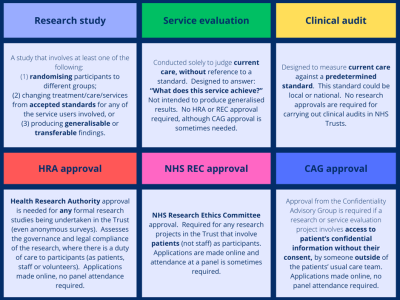
Still unsure? Please visit the HRA decision tool here and answer a short series of questions and the tool will let you know whether or not your study is research.
IWT/PHU Research Standard Operating Procedures are now stored on the Research instance of Ideagen Quality Management (IQM), our quality management system. Please access SOPs via this system to ensure you are utilising the correct version.
If you are unable to access this system, please contact pho-tr.




